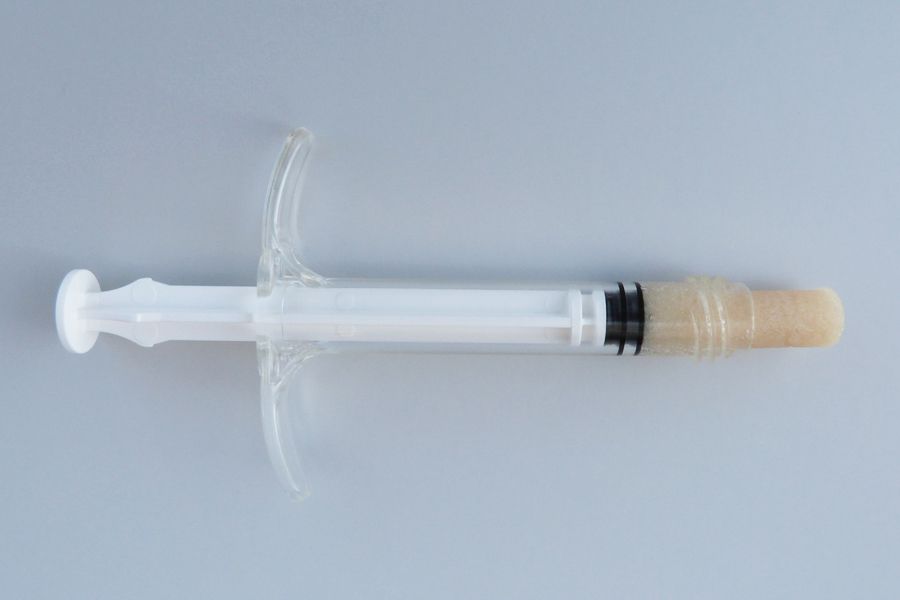
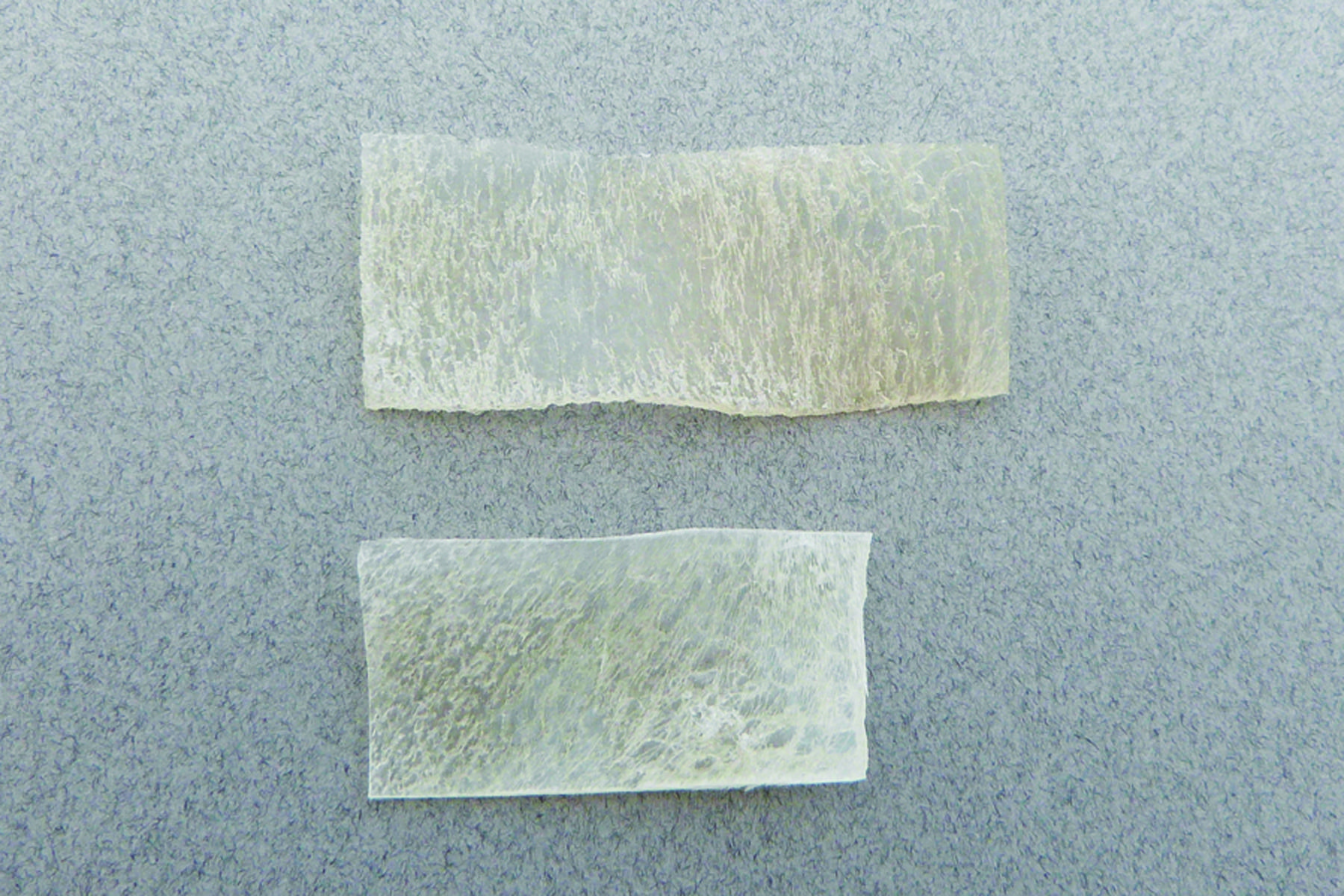
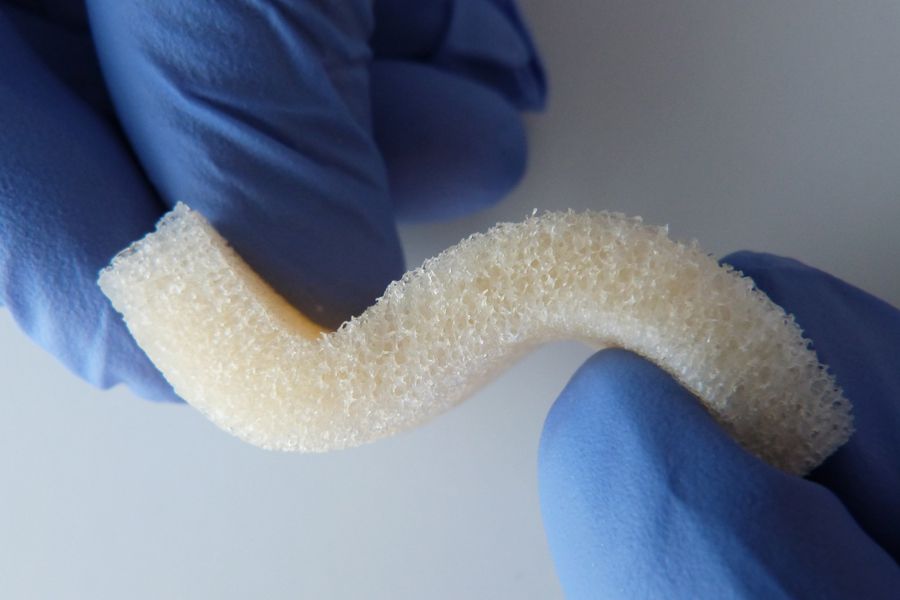
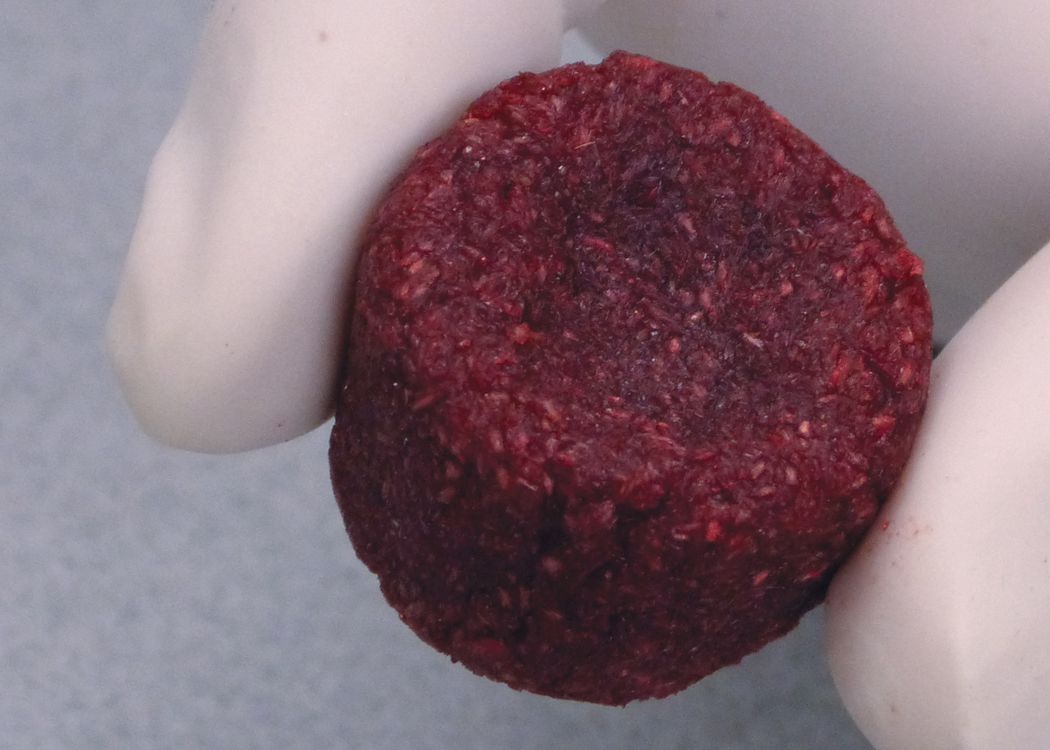
Standard Medical Equipment Systems, LLC – DBA, (“Standard Med” ) is a certified minority (MBE) Veteran (SDVOSB) owned company, certified by the Center for Veteran Enterprises, (CVE). Standard Med was established by Anthony B. Goodesmith, in the year 2005, to deliver high quality medical devices and laboratory equipment. Mr. Goodesmith served as a highly decorated combat signaler/communications officer in the U.S. Army and New Jersey National Guard for over 18 years. His units took part in Operation Desert Storm, and several other military campaigns after the 9/11 U.S. Terror Attacks (Operations Enduring Freedom and Noble Eagle). He received his B.S. in Marketing and Media Arts from Long Island University, NY, and his Master’s degree in Biotechnology Management from The University of Maryland University College (UMUC).Mr. Goodesmith has been working in the health care, medical industry in sales and management for over 20 years at several Fortune 500 pharmaceutical and medical device companies. Such as Eli Lilly & Company, Solvay Pharmaceuticals, AstraZeneca, Inc., Roche Laboratories, Inc. and now Standard Medical Systems, LLC. He has received the Elite Salesman Award, President’s Award, U.S. Congressional VIP Citation, and achieved #1 status in selling various pharmaceutical drugs and medical devices throughout his career. Mr. Goodesmith shares his tremendous expertise as a staff writer for the DC Health-Technology Examiner, based on his exceptional knowledge about the epidemiology of health-related issues and via medical device, regenerative, biological solutions. You may reach Mr. Goodesmith at his corporate email address: tony@standardmed.us. We pride ourselves on our distinct array of medical devices and diagnostic equipment we offer to the healthcare community, which delivers the highest quality control, safety and efficacy with the latest breakthroughs in medical technology.